May 7, 2021
A welcome new wrinkle on FDA’s MOU
FDA has launched a new web page to help answer questions regarding the MOU. Of note is point number 6, which is new information:
Can a state that is prohibited by a state law from disclosing a complainant’s name and contact information fulfil the agreed upon data reporting under the MOU?
Yes. Under the MOU, the state is agreeing to report the name and contact information of the complainant, if available, to FDA. If providing this information is prohibited by state law, FDA does not consider it to be “available” for purposes of the MOU.
That could make a difference for states such as Florida that have indicated concern that the MOU conflicts with their confidentiality laws.
Which states have signed? Will sign? Won’t? Here’s the latest map from NABP (click to enlarge:)
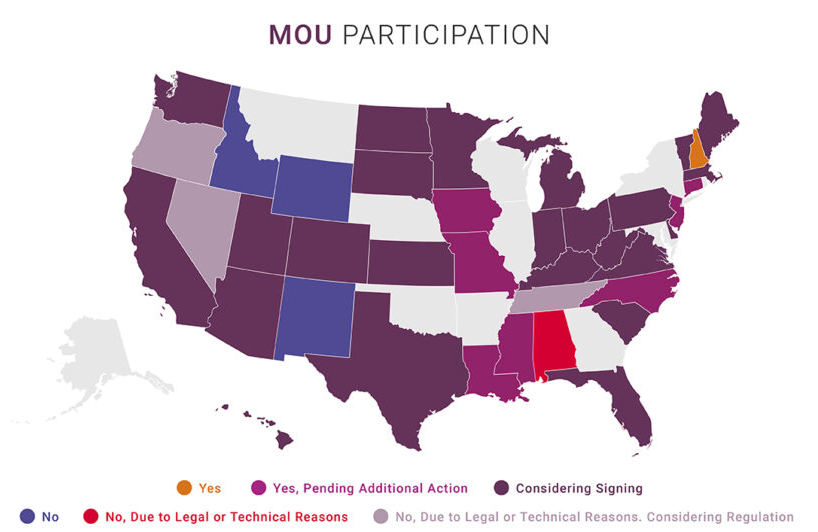
(It’s also available on the NABP website.)
REMINDER: While the MOU is flawed, APC’s position is that compounders need to reach out to their board of pharmacy now and urge the board to determine if it can or will sign. Several states have discovered that state law prohibits them from signing.
If your state determines it can sign, urge it to do so before the October 26 deadline. If it determines it cannot sign — and it’s best to determine that now, and not at the last minute — the board of pharmacy needs to contact FDA immediately and urge the agency to extend the signing deadline. Ultimately, it’s better for states to sign the MOU if they can, than to have a catastrophic five-percent cap imposed on out-of-state shipments of compounded preparations.
APC members briefed on MOU reporting
APC held a virtual briefing Thursday with Melissa Madigan of NABP. She explained how reporting would likely work (including what information would be gathered and how it would be used) in states that sign the MOU and choose to use NABP’s online reporting tool. She also demonstrated that tool — the Information Sharing Network (ISN) — which states can choose to use for their reporting to FDA under the MOU.
The system will collect or calculate compounded-prescription information and notify state boards about inordinate amounts shipped out of state — that is, more than 50 percent of compounded prescriptions in states that have signed the MOU. Boards will then be required to pass that information to FDA. Note that it is perfectly legal to ship that much out of state, but it may trigger a closer look by the BoP or FDA.
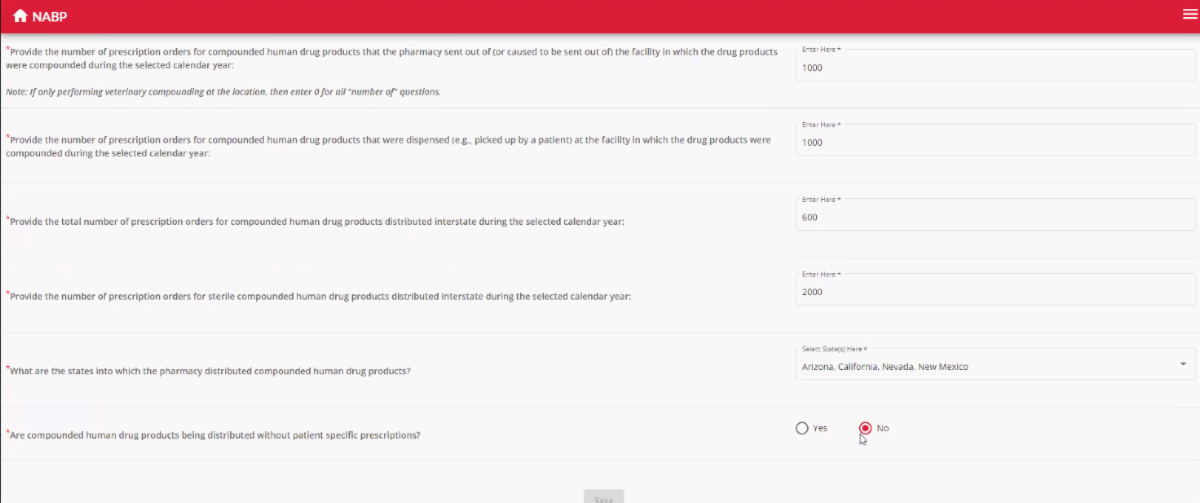
Compounding pharmacies will need to submit their prescription information annually. If their state chooses to use NABP’s ISN, it can be done through the pharmacy’s existing NABP e-Profile and will look like this (click to enlarge):
(Currently the ISN is manual — it does not connect with pharmacy software platforms, although Madigan said it would consider building such a connection.)
Anticipating more questions, NABP has built a website full of information about the MOU and how information will be shared — it also answers a lot of questions about the process.
Missed APC’s briefing yesterday? No worries. We’ve made it available for viewing here.







![Topi-CLICK a Division of TEAM Outlines[1]](https://a4pc.org/files/Topi-CLICK-a-Division-of-TEAM-Outlines1.png)